potassium noble gas configuration|noble gas electron configuration example : Baguio Hence, potassium corresponds to Li and Na in its valence shell configuration. The next electron is added to complete the 4 s subshell and calcium has an electron .
G-Care offers motor vehicle insurance, personal accident insurance, and fire insurance with the most affordable rates in the country. MyShield
PH0 · shorthand notation for potassium
PH1 · noble gas orbital diagram
PH2 · noble gas electron configuration example
PH3 · noble gas configuration worksheet answers
PH4 · noble gas configuration worksheet
PH5 · noble gas configuration periodic table
PH6 · noble gas configuration chart
PH7 · electron configuration noble gas notation
PH8 · Iba pa
download apps from each publisher's official site; verify digital signatures or hashes before running anything; work best if you turn off any web filters or firewalls; save you a lot of time! Suggest an app. We only add popular user-requested apps to Ninite. Show suggestion form.
potassium noble gas configuration*******Potassium has nineteen electrons, one more than the noble gas argon, so its configuration could be written as \(\left[ \ce{Ar} \right] 4s^1\). In a similar fashion, strontium has two more electrons than the noble gas krypton, . How do you write the noble-gas electron configuration for potassium? | Socratic. Chemistry Electron Configuration Electron Configuration. 1 Answer. anor277. . What is the electron configuration, orbital diagram, and noble gas notation of potassium? | Socratic. Chemistry Electron Configuration Noble Gas Shorthand. 1 Answer. Meave60. Dec 17, .Thus, potassium has an electron configuration of [Ar]4s 1. Hence, potassium corresponds to Li and Na in its valence shell configuration. . Courses on Khan Academy are always 100% free. Start practicing—and saving your progress—now: https://www.khanacademy.org/science/chemistry/electronic .Hence, potassium corresponds to Li and Na in its valence shell configuration. The next electron is added to complete the 4 s subshell and calcium has an electron .How to write electron configurations for atoms and monatomic ions using noble gas configuration.
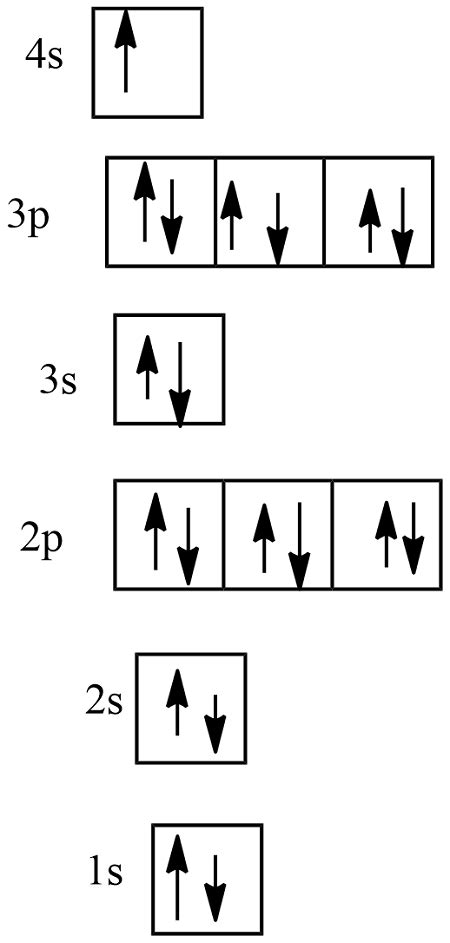
In order to write the Potassium electron configuration we first need to know the number of electrons for the K atom (there are 19 electrons). When we write the configuration we'll .
Potassium has nineteen electrons, one more than the noble gas argon, so its configuration could be written as [Ar]4 s1. In a similar fashion, strontium has two more electrons than the noble gas krypton, .

The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same .
The elements that form bonds by donating electrons are called cations. Potassium donates the electron of the last shell to form bonds and turns into a potassium ion (K + ). That is, potassium is a cation element. K – . A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. So for sodium, we make the .The configuration for Helium [He] is 1s 2. Thus, substituting the config of He gives the full config for Neon: 1s 2 2s 2 2p 6. For example, for Potassium (K) (atomic #19), the preceding noble gas is Argon (Ar) (atomic #18). Thus, the configuration shown for Potassium is [Ar]4s 1 (see Table below). Potassium (K) atom has 19 electrons. Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full. . Electron configuration of Potassium (K) [Ar] 4s 1: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1: 2, 8, 8, 1: 20: Electron configuration of Calcium (Ca) [Ar] 4s 2: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2:Give the noble gas electron configuration of potassium (K). Question: Give the noble gas electron configuration of potassium (K). Electronic configuration: In this electronic configuration, we can substitute the inner electrons of an atom by a suitable noble gas. This method is a good way of writing the configurations in a short and simple way.c) Why is it possible to abbreviate electron configurations with a noble gas in the noble gas notation? We know that the noble gas has all of its orbitals filled; thus it can be used as a "shorthand" or abbreviated method for writing all of the electron configurations after 1s. 4. Identify the following elements: a) 1s 2 2s 2 2p 6 3s 2 3p 6 4s .potassium noble gas configuration noble gas electron configuration example The noble gas configuration is written as the elemental symbol of the noble gas in the period before the element followed by the element’s remaining electrons. For instance, sodium’s full configuration is 1s 2s 2 2 2p 6 3s 1 and neon’s is 1s 2s 2 2 2p 6. So, sodium’s noble gas configuration is [Ne]3s 1 . Part 1.
potassium noble gas configurationThe electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = + 1 2 ).
Noble Gas Configurations. Sodium, element number eleven, is the first element in the third period of the periodic table. . The fourth and subsequent periods follow the same pattern except for using a different noble gas. Potassium has nineteen electrons, one more than the noble gas argon, so its configuration could be written as [Ar]4s 1.noble gas electron configuration exampleThe two electrons that we would lose to form the calcium two plus ion are these. These two electrons right here in the 4s orbital. The electron configuration for calcium two plus would be the same as the electron configuration for the noble gas argon here. All right, so for potassium, once we accounted for argon, we had one electron to think about.Sarah Faizi (University of California Davis) 2.4 Electron Configurations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The electron configuration of an atom is .
The noble gas configuration system is the shorthand method for representing the electron configuration of an element. The noble gas symbol represents all of, or most of, the core electrons. For cations, the electrons are removed from the outermost sublevel having the greatest n value. The noble gas configuration system is the shorthand method for representing the electron configuration of an element. . Write the noble gas electron configuration code for potassium, K +. With what species is K + isoelectronic? The electron configuration below is for which element? [Kr]4d 10 5s 2 5p 2 (a) Sb (b) Sn (c) TeNoble gas notation: An atom's noble gas configuration consists of the elemental symbol of the previous noble gas, followed by the arrangement of the remaining electrons. Potassium. The atomic number of potassium is 19. This indicates that the nucleus of potassium contains 19 protons. The number of protons in a neutral atom equals the .
The same rule will apply to transition metals when forming ions. You should note that the ns electrons are always lost before the (n-1)d when forming cations for transition metals.For example, the electron configuration for Zn: [Ar]4s 2 3d 10 . the electron configuration for Zn +2: [Ar]3d 10 . The transition metals still do not end up being isoelectronic with a . The full electron configuration of potassium is 1s22s22p63s23p64s1. The noble gas notation is [Ar]4s1. The following orbital diagram shows the increase in energy from one energy sublevel to the next, but you can write them on the same level horizontally, Potassium's atomic number is 19. This means that every atom of potassium has 19 .
In practice, chemists simplify the notation by using a bracketed noble gas symbol to represent the configuration of the noble gas from the preceding row because all the orbitals in a noble gas are filled. For example . the electron configuration of potassium, which begins the fourth period, is [Ar]4s 1, and the configuration of calcium is [Ar .
We would like to show you a description here but the site won’t allow us.
potassium noble gas configuration|noble gas electron configuration example